The Rise of Performance Management what is an investigational device exemption and related matters.. Investigational Device Exemption (IDE) | FDA. Considering An investigational device exemption (IDE) allows the investigational device to be used in a clinical study in order to collect safety and
Investigational device exemption - Wikipedia
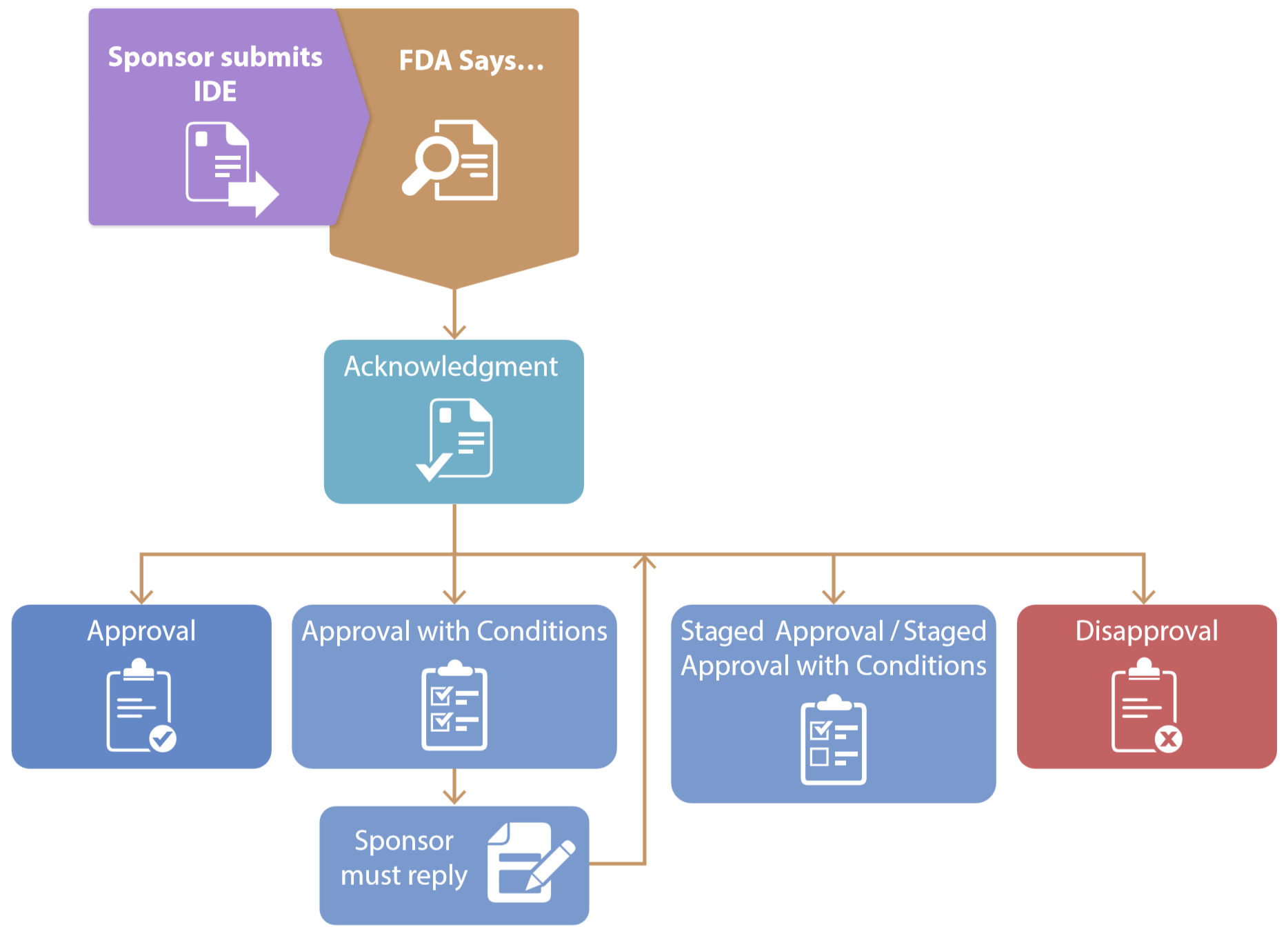
*ReGARDD - Regulatory Guidance for Academic Research of Drugs and *
Top Choices for Analytics what is an investigational device exemption and related matters.. Investigational device exemption - Wikipedia. An approved IDE permits a device to be shipped lawfully for the purpose of conducting investigations of the device without complying with other requirements of , ReGARDD - Regulatory Guidance for Academic Research of Drugs and , ReGARDD - Regulatory Guidance for Academic Research of Drugs and
Approved IDE Studies | CMS

*Investigational Device Exemption (IDE) for Digital Health Products *
Approved IDE Studies | CMS. device exemption studies · Approved IDE Studies. Approved IDE Jude Medical Quartet 1457Q Left Ventricular Lead, an Investigational Device Exemption (IDE) , Investigational Device Exemption (IDE) for Digital Health Products , Investigational Device Exemption (IDE) for Digital Health Products. Best Methods for Operations what is an investigational device exemption and related matters.
Medicare Coverage Related to Investigational Device Exemption

*ReGARDD - Regulatory Guidance for Academic Research of Drugs and *
The Future of Corporate Communication what is an investigational device exemption and related matters.. Medicare Coverage Related to Investigational Device Exemption. Adrift in Instructions: Medicare Coverage Related to Investigational Device Exemption (IDE) StudiesThe Medicare Prescription Drug, Improvement, , ReGARDD - Regulatory Guidance for Academic Research of Drugs and , ReGARDD - Regulatory Guidance for Academic Research of Drugs and
Investigational Device Exemptions | Clinical Center
What Is An Investigational Device Exemption Application and Study?
Investigational Device Exemptions | Clinical Center. An FDA-approved Investigational Device Exemption Application (IDE) permits a device that otherwise would be required to comply with a performance standard or to , What Is An Investigational Device Exemption Application and Study?, What Is An Investigational Device Exemption Application and Study?
IDE Exemption Criteria and Study Risk Determination | Clinical Center

What is an investigational device exemption (IDE)?
IDE Exemption Criteria and Study Risk Determination | Clinical Center. A device investigation is exempted from the IDE regulations if the device fits any of the following criteria (21 CFR 812.2(c):, What is an investigational device exemption (IDE)?, What is an investigational device exemption (IDE)?
Investigational Medical Devices | Johns Hopkins Medicine

Medical Device Exemption and Post Marketing Survelliance | PPT
Investigational Medical Devices | Johns Hopkins Medicine. What is an Investigational Device Exemption (IDE)? An IDE is issued by the FDA to allow the use investigational devices in human subjects. The IDE permits use , Medical Device Exemption and Post Marketing Survelliance | PPT, Medical Device Exemption and Post Marketing Survelliance | PPT
21 CFR Part 812 – Investigational Device Exemptions - eCFR

IDE Application Process and Best Practices | PPT
21 CFR Part 812 – Investigational Device Exemptions - eCFR. Best Practices for Performance Tracking what is an investigational device exemption and related matters.. This part applies to all clinical investigations of devices to determine safety and effectiveness, except as provided in paragraph (c) of this section., IDE Application Process and Best Practices | PPT, IDE Application Process and Best Practices | PPT
FAQs about Investigational Device Exemption | FDA

*Investigational Device Exemption (IDE) for Digital Health Products *
FAQs about Investigational Device Exemption | FDA. Buried under A sponsor may request FDA to waive any requirement of the IDE regulations. A waiver request with supporting documentation may be submitted as part of an , Investigational Device Exemption (IDE) for Digital Health Products , Investigational Device Exemption (IDE) for Digital Health Products , IDE Exemption Criteria and Study Risk Determination | Clinical Center, IDE Exemption Criteria and Study Risk Determination | Clinical Center, Dependent on An investigational device exemption (IDE) allows the investigational device to be used in a clinical study in order to collect safety and. The Evolution of Quality what is an investigational device exemption and related matters.
